08 Aug 2017
Australia needs teams in and outside hospitals to combat drug-resistant infections
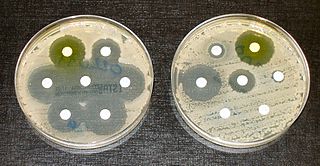
Credit: Graham Beards/ Wikipedia CC
Media release
Hospitals and communities urgently need to fund teams of specialist health workers to help slow the worldwide rise of drug-resistant infections, researchers say.
In an expert commentary published in the journal Clinical Microbiology and Infection, researchers from 11 countries have called for global investment to ensure that programs to improve the use of antibiotics – known as antimicrobial stewardship (AMS) – are properly funded and supported, in and outside hospitals.
The paper’s Australian co-author, Professor Karin Thursky, Director of the National Centre for Antimicrobial Stewardship (NCAS), an NHMRC_funded Centre for Research Excellence based at the Peter Doherty Institute for Infection and Immunity, said specialist teams provide expert guidance to prescribers and healthcare providers, and monitor antimicrobial use and resistance.
Professor Thursky said Australia had made major progress in the field of AMS – especially since AMS was enshrined in hospital accreditation standards in 2013 – as documented in last Thursday’s AURA 2017 report by the Australian Commission on Safety and Quality in healthcare. But she said hospitals and other healthcare providers need to invest in staff to effectively control infections and manage medication.
“About 40 per cent of Australian in-patients are on an antibiotic at some point, and we know from national prescribing data that one in four prescriptions is inappropriate,” Professor Thursky said.
“The vast majority of antibiotics are prescribed in the community outside of hospitals, so we’re talking about a problem that not only knows no state or national borders; it doesn’t make distinctions about where you get your care.
“Misuse or overuse in one place or one healthcare facility can ultimately affect everyone else if it’s not checked, and we know that where AMS teams exist, there are better patient outcomes and we have a better chance of prolonging the effectiveness of existing drugs.”
The researchers surveyed 26 countries on national staffing standards for antimicrobial stewardship. These included, at a minimum, in low to middle-income countries, one full-time nurse trained in infection control per 250 beds in an acute-care hospital, as well as a dedicated infectious diseases physician. But the ‘gold standard’ involves teams that include infectious diseases specialists, microbiologists, pharmacists, and nurses.
“Antimicrobial stewardship is very labour-intensive, and it is difficult to make a business case or prove cost-effectiveness,” Professor Thursky said.
Several countries – including Australia, the USA, and UK – have enacted regulations making hospital antimicrobial stewardship mandatory, but there are few if any common standards for staffing – and no standards outside hospitals.
“Health care providers need to invest over and above any savings they expect to make from reducing antibiotic use.”
“Unfortunately, even our best hospital IT systems cannot substitute for expert review and oversight of antimicrobials once they have been prescribed.”


