10 Mar 2020
Cluing in on Q-fever
The immune system has multiple defence mechanisms to fight infection, but some bacteria take advantage of these.
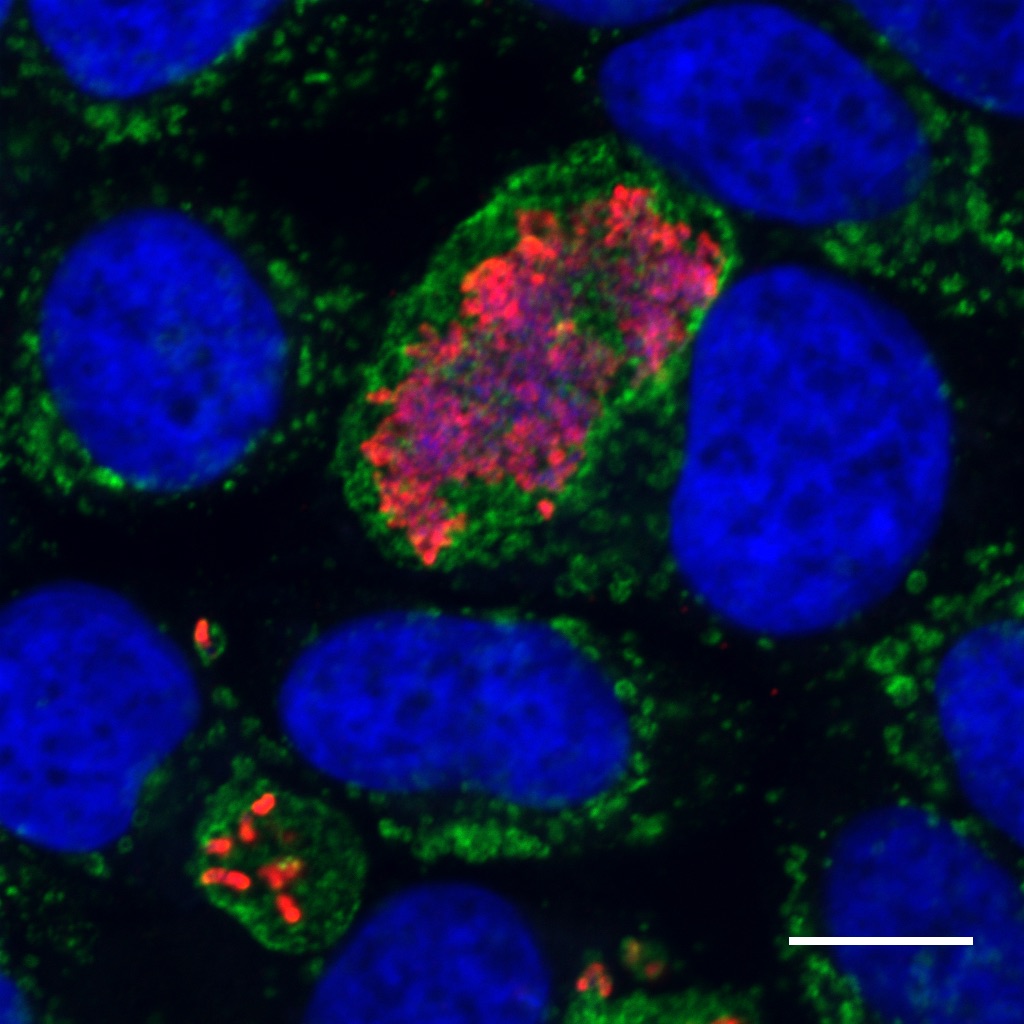
Coxiella (red) replicating within a membrane bound vacuole (green) inside a human cell (nucleus = blue). Courtesy Dr Hayley Newton.
Written by Doherty Institute PhD student Catriona Nguyen-Robertson.
Coxiella burnetii is one such bacterium that leverages our defences to its own advantage. Researchers based at the at the Peter Doherty Institute for Infection and Immunity (Doherty Institute) have published a study in PNAS that provides insight into how this unique bacterial pathogen takes over cells.
C. burnetii is commonly found in domestic, wild, and livestock animals. It can spread from infected animals to humans to cause Q fever, often through inhalation of contaminated dust and exposure to livestock. Infection triggers abortions in goats and sheep and causes flu-like symptoms in humans that can progress to pneumonia.
Q fever is becoming alarmingly prevalent in the Australian agricultural industry as a result of drought and is the most reported animal-to-human infection in the country.
Adding to the challenge, C. burnetii is a highly infective and efficient pathogen that can survive for months to years in the environment. It is resistant to heat, drying and many common disinfectants, allowing it to survive for long periods in the environment and the wind can disperse the pathogen over several kilometres.
Given that traditional decontamination methods are ineffective against this pathogen, it is imperative to understand it on a cellular level and determine what makes it pathogenic (disease-causing).
A study led by the University of Melbourne’s Dr Patrice Newton and Dr Hayley Newton from the Doherty Institute, identified host mechanisms that the pathogen exploits to establish infection.
“It isn’t just about understanding bacteria. Our study highlights the importance of interactions between a host and pathogen,” Dr Hayley Newton, senior author of the study said.
“We aimed to answer the fundamental question of what makes bacteria pathogenic and learned more about our own cell biology in the process.”
Upon infection, many bacteria will rapidly employ mechanisms to stop the host cell from killing them. C. burnetii, however, bides its time.
The host cell attempts to degrade bacteria by creating a hostile, acidic environment that is typically lethal, but C. burnetii instead uses this environment as a trigger to engage its weapon: a syringe-like structure that pumps out its cargo and machinery into the host cell. This allows it to completely take over the host cell to produce more bacteria that can infect new cells.
Associate Professor Kaylene Simpson, head of the Victorian Centre for Functional Genomics at the Peter MacCallum Cancer Centre provided the robotic infrastructure, reagents and technical expertise to enable the Newton lab to assess the impact of every gene in the genome to identify mutants with reduced pathogenicity.
“This was a really exciting project with such a major opportunity to understand the infection process and to drive change in the future in the agricultural sector, we were delighted to be a part of it,” said Associate Professor Simpson.
The genes lost in mutants with reduced pathogenicity would therefore be essential for the host processes that enable the bacterium’s infection. Using this method, the researchers identified multiple factors that are involved in the creation of a hostile environment that typically kills bacteria.
“Not only has this study further enhanced our understanding of C. burnetii pathogenesis, but we can also learn about the host-pathogen interactions that contribute to bacterial infection and the different environmental triggers that pathogens can sense to facilitate their pathogenicity,” Dr Newton said.
This research and the techniques used in this study can now be applied to other bacteria and are also useful as a way of learning more about our own cell biology.
C. burnetii infection poses a threat to local agricultural and meat processing industries and affects many rural communities.
Vaccinations for Australian livestock are costly, but the cost of inefficient public health strategies is also great. The health and economic burden of Q fever was demonstrated by the culling of 50,000 dairy goats following the outbreak in the Netherlands from 2007-2010, which caused more than 4,000 human cases of the disease.
This research takes several steps forward in being able to understand the bacterium and learning alternative ways to manage and control it in the face of a rise in Q fever cases.


