10 May 2021
Identifying the degree or active of inactive COVID-19 infection
By investigating how SARS-CoV-2 (the virus that causes COVID-19) infects cells, Doherty Institute researchers have identified what may be a way of identifying the degree to which a COVID-19 infection is active or inactive and published the findings today in Cell Reports.
SARS-CoV-2 uses expression of subgenomic RNA within host cells to produce viral proteins that enable it to replicate, assemble new viral particles and evade the immune response, enabling it to further infect new cells.
Because SARS-CoV-2 is a large viral genome, it needs to find shortcuts in how it turns its genetic code into the actual molecules that cause infection.
It does this by producing ‘subgenomic RNA’ which are truncated versions of the full genome, which are then copied in large numbers to produce the transcriptome. It’s a bit like printing out the first and last chapter of a book, then photocopying this many times over.
It means a lot of the information is left out of the smaller subgenomic RNA, but you end up with many more copies of the most important information.
University of Melbourne Professor Lachlan Coin, laboratory head at the Doherty Institute, and his team used RNA sequencing on SARS-CoV-2-infected human and monkey cells at three timepoints (over 48 hours) to determine when subgenomic mRNA was being actively produced.
Early on – at two hours post infection – subgenomic mRNA was virtually undetectable in the human cell lines but comprised about 14 per cent of all RNA in the monkey cell line. In all cell lines, however, the proportion of subgenomic RNA versus total RNA peaked at 24 hours post-infection, falling again slightly at 48 hours.
“We hope in the future our work might help distinguish patients who have long-term positivity for the virus but who are non-infectious from those who remain infectious. But that is future work that still needs to be done,” Professor Coin explained.
“This work will enable the development of new diagnostic tests for monitoring the progression of SARS-CoV-2 infectious cycle.
“The tools we developed for our investigations may also assist in better understanding the mechanism-of-action of therapeutic agents as well as monitoring the efficiency of the immune response to SARS-CoV-2 in vaccination studies.”
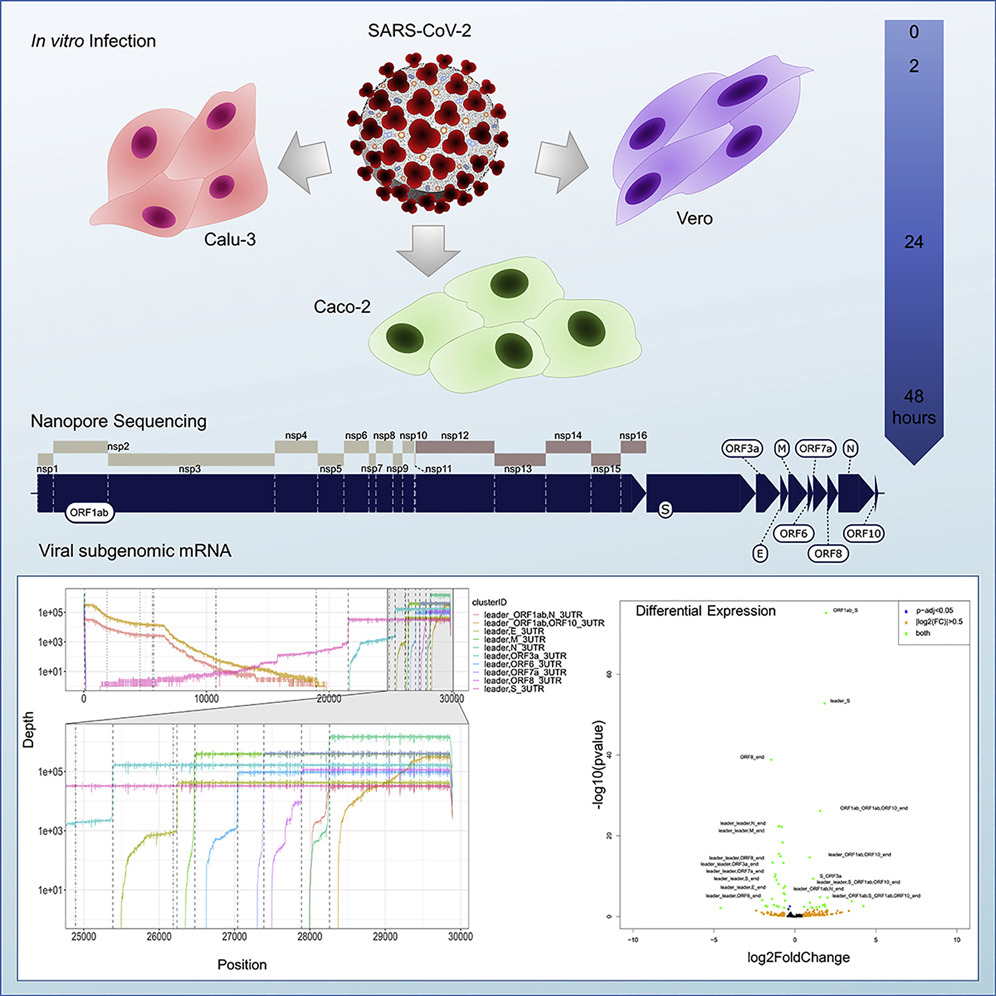
Graphical abstract


