09 Sep 2022
Vaccine expected to induce strong immune responses against the 2022 monkeypox virus, research shows
New research has suggested that recommended vaccinia virus (VACV)-based vaccines will mount a robust immune response against the monkeypox virus observed in the current outbreak (MPXV-2022).
Since the new virus was first observed in early May 2022, over 52,000 cases have been confirmed in more than 90 countries, including Australia, where 124 cases have been diagnosed (confirmed and probable).
The study, co-led by University of Melbourne Professor Matthew McKay, ARC Future Fellow and Honorary Professor at the Peter Doherty Institute for Infection and Immunity, and Professor Ahmed Abdul Quadeer, Research Assistant Professor at the Hong Kong University of Science and Technology, was published in the international journal Viruses.
Weeks after the new strain emerged, the team undertook genomic research to find out if the genetic mutations observed in MPXV-2022 may affect vaccine-induced immune responses against monkeypox.
![Figure 1. Mapping mutations observed in 2022 monkeypox viruses (MPXV-2022) and Congo Basin clade of monkeypox (MPXV-CB) on the structure available for vaccinia virus (VACV) surface proteins: (A) H3L [PDB ID: 5EJ0] and (B) D8L [PDB ID: 4E9O].](https://uat.doherty.edu.au/uploads/content_img/Fig1.jpg)
Figure 1. Mapping mutations observed in 2022 monkeypox viruses (MPXV-2022) and Congo Basin clade of monkeypox (MPXV-CB) on the structure available for vaccinia virus (VACV) surface proteins: (A) H3L [PDB ID: 5EJ0] and (B) D8L [PDB ID: 4E9O].
“Specific VACV-based vaccines have demonstrated high efficacy against monkeypox viruses in the past and are considered an important outbreak control measure,” Professor McKay said.
“However, given this is a novel monkeypox virus, we still lack scientific data on how well human immune responses triggered by VACV-based vaccines will recognise MPXV-2022 and provide protection against disease.”
Using genomic and immunological data, the team evaluated the genetic similarities and differences between VACV and MPXV-2022, specifically within the protein regions that are targeted by vaccine-induced neutralising antibodies or T cells.
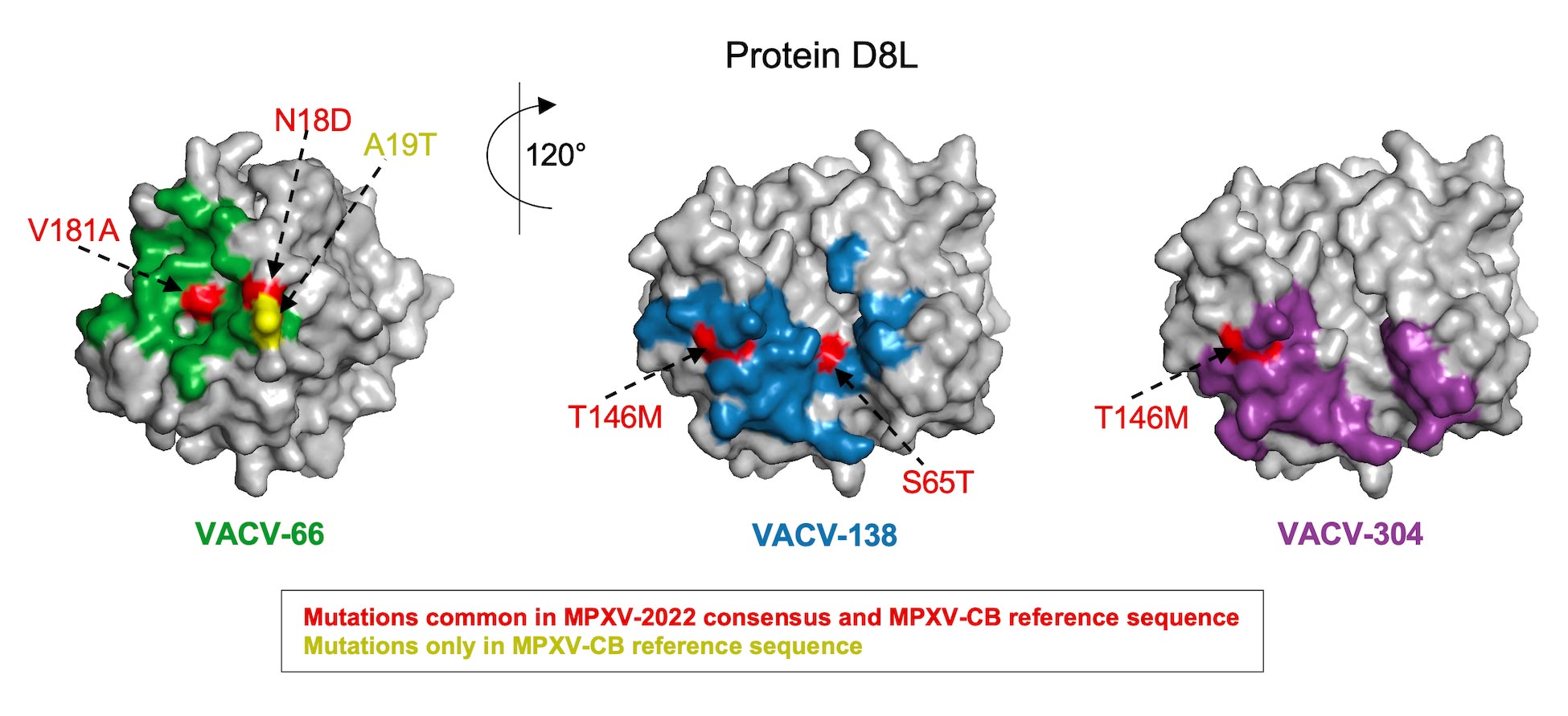
Figure 2. MPXV-2022 does not comprise any new mutations relative to the MPXV-CB reference sequence in the epitopes of the three known neutralizing antibodies (VACV-66, VACV-138, and VACV-304) in the D8L protein of VACV.
“While we identified a small number of distinct mutations in MPXV-2022, our study more broadly demonstrates that VACV and MPXV-2022 are highly genetically similar in the regions targeted by the immune system through vaccination,” Professor McKay said.
Professor Quadeer said that the findings are reassuring.
“Based on our analysis, we anticipate that the immune responses generated by VACV-based vaccines would continue to do a good job of recognising and responding to MPXV-2022, as was the case for monkeypox viruses in the past. Our data lends further support to the use of vaccines being recommended globally for combating MPXV-2022,” Professor Quadeer said.
Professor McKay said: “While bringing together sequencing and immunological data provides evidence to anticipate a strong immune response, clinical studies are required to determine the exact efficacy of these vaccines against MPXV-2022.”
Funding: This research was supported by the General Research Fund of the Hong Kong Research Grants Council and through the Australian Research Council Future Fellowship, funded by the Australian Government.
Preview image: False coloured electron micrograph of a single negative-stained Monkeypox virus particle. Negative contrast transmission electron microscopy using 2% phosphotungstic acid, 52,000x magnification. Image credit: Dr Jason Roberts, Head of Electron Microscopy and Structural Virology at The Royal Melbourne Hospital's Victorian Infectious Diseases Reference Laboratory, Doherty Institute.


