Hepatitis C is treatable, with direct-acting antivirals (DAAs) available on the Pharmaceutical Benefits Scheme requiring only 8-12 weeks of daily tablets to achieve a cure.
What to do if hepatitis C management is indicated
- Confirm the patient’s hepatitis C status
- Confirm that hepatitis C management has not been undertaken elsewhere
- Follow the ashm guidance for decision-making in HCV OR
- Refer to the guidance in:
- HealthPathways Gippsland (for clinics in Gippsland PHN)
- HealthPathways Melbourne (for clinics in Eastern Melbourne PHN and North Western Melbourne PHN)
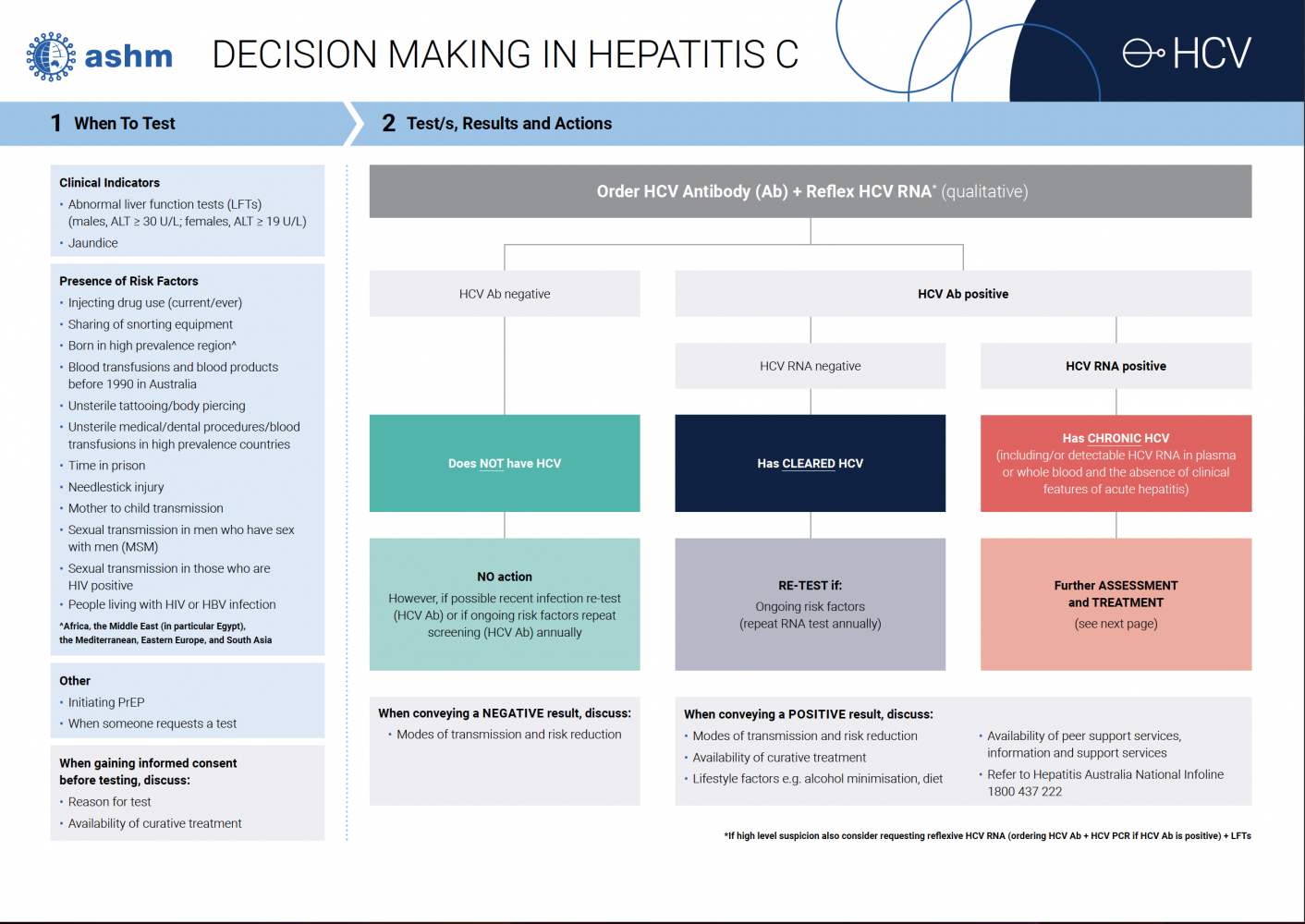
Summary of testing to initiate hepatitis C management
For patients who are HCV antibody positive, order:
- HCV RNA (qualitative)
- LFTs
For patients who are HCV RNA positive, order:
- Full Blood Count
- Urea, electrolytes, creatinine
- LFTs (including AST) and INR
And assess liver fibrosis through APRI or FibroScan®
Test for anti-HAV (Hepatitis A immunity) and coinfection Hepatitis B, and HIV if there is no previous record of testing
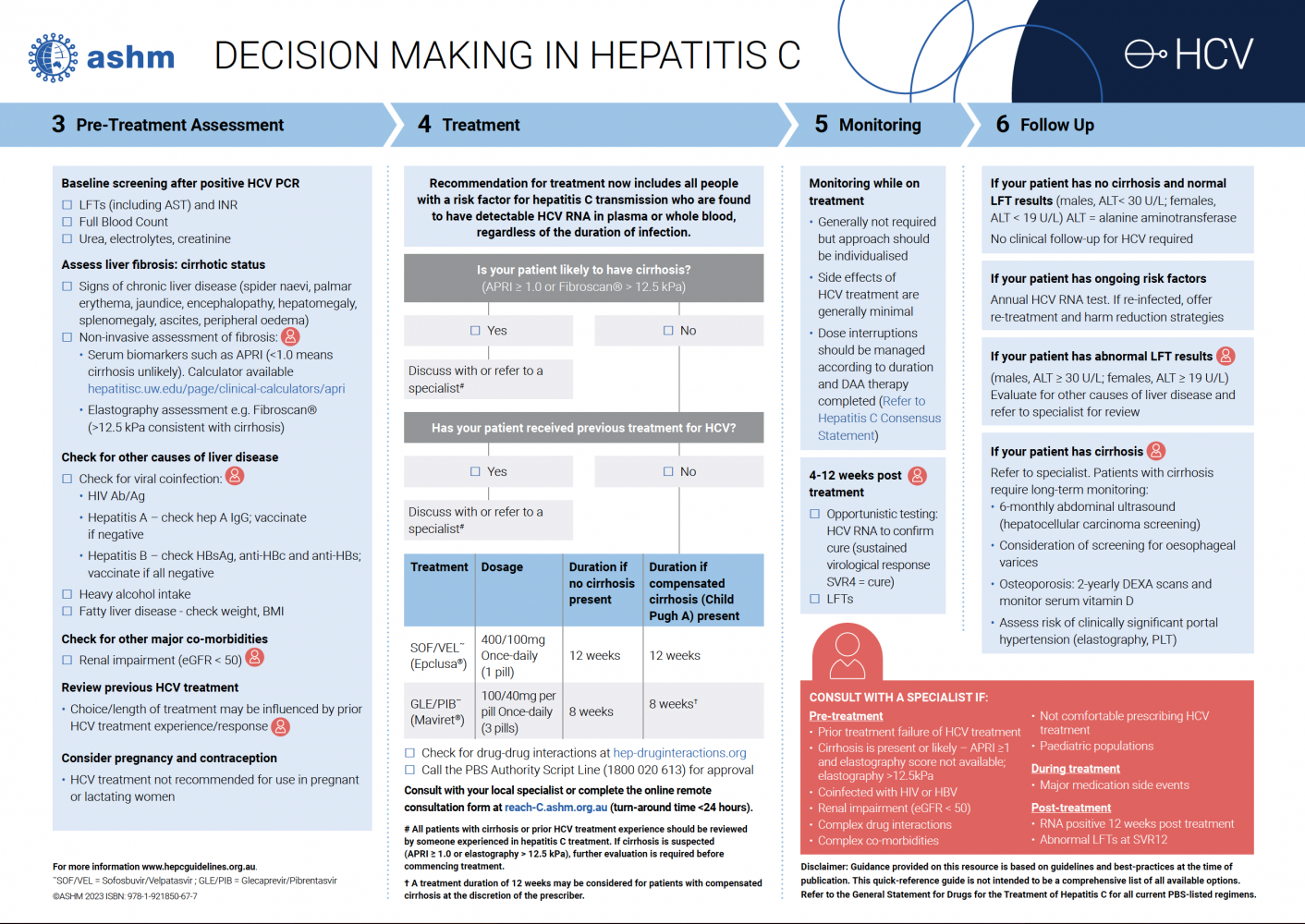
Undertake a full pre-treatment assessment, in accordance with the ashm guidance for decision-making in HCV before initiating treatment.

The HepLOGIC WALRUS risk tool
The information on this page supports the use of the POLAR/WALRUS viral hepatitis risk tool that is being piloted in the HepLOGIC pilot and feasibility study. WALRUS provides a testing alert when a patient may be living with chronic hepatitis C virus (HCV) but has no evidence of treatment.
The hepatitis C management alert in WALRUS is triggered by ANY of the following in a patient record:
- A hepatitis C diagnosis or positive hepatitis C antibody result AND/OR
- HCV nucleic acid testing (HCV PCR) ever having been ordered
AND no there is evidence that hepatitis C treatment has previously been prescribed.
Refer to ashm's quick reference guide Decision-making in HCV for best practice guidance on management.